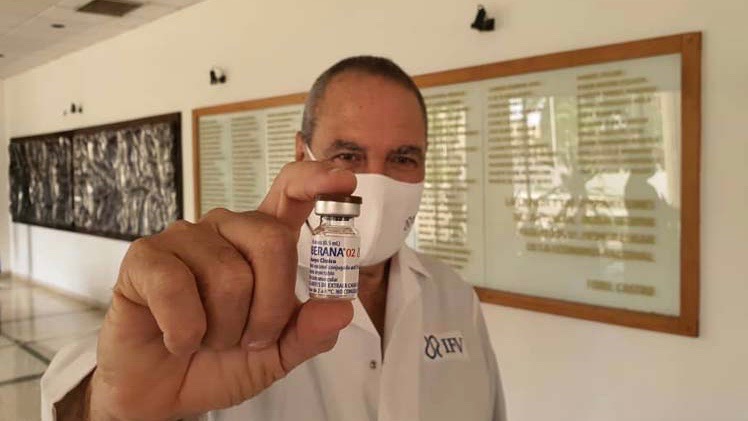
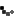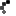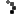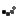Cuba’s vaccine candidate for COVID-19 Soberana 02 of the Finlay Vaccine Institute (IFV) began its third phase of clinical trials on March 4. A day before, on March 3, the country’s Center for State Control of Medications, Medical Equipment and Devices (CECMED) authorized the IFV to begin phase III trials of the vaccine, after an exhaustive evaluation of the preclinical and clinical trial findings.
The director of the CECMED, Olga Lidia Jacobo Casanueva, said that “the vaccine candidate has shown an adequate safety profile, and has passed all the reviews done by the CECMED during phase I and II studies.” Jacobo Casanueva added that “people who have taken part in trials have developed neutralizing antibodies, which means the vaccine is effective against the virus, therefore it is encouraging news for the population. However, we must be patient and comply with established regulations.”
The fundamental objective of the phase 3 trials of Soberana 02 is to evaluate the efficacy, safety and immunogenicity of the vaccine. In this decisive phase, the research will be multicenter, adaptive, in parallel groups, randomized, controlled with placebo and double blind.
According to the Cuban public health ministry, the clinical trial will be carried out in 50 vaccination centers distributed in eight municipalities of the capital Havana: Plaza, Playa, Centro Habana, Diez de Octubre, Cerro, Habana Vieja, La Lisa and Marianao. 44,010 volunteers between the ages of 19 and 80 will participate in the study and will receive two doses of the Soberana 02 and a booster dose of the Soberana 01.
Soberana 02 and Soberna 01 both are being developed by the IFV. Recently, the institute announced a new vaccine candidate, Soberana 01-A, which is the institute’s third and the country’s fifth vaccine candidate. It is explicitly meant for people convalescent from the disease. It seeks to reduce the risk of new contagion in patients who have already been infected with the disease.
On March 3, Cuban President Miguel Díaz-Canel announced the news regarding the fifth vaccine candidate and welcomed the advances in science. “Another milestone for our science: Soberana 01-A is a new vaccine candidate (the fifth of Cuba) for convalescents from COVID-19. The news was given by the creators of the vaccine in the weekly expert briefing session. Cuban Science keeps giving us good news,” tweeted the head of state.
Another hit of our Science: #Soberana01A is a new vaccine candidate (the 5th of #Cuba) for #COVID19 convalescents. The news was given by the creators of the vaccine in the weekly expert briefing session. Cuban Science keeps giving us good news. #SomosCuba #LivingCuba pic.twitter.com/2wPjBlanrW
— Miguel Díaz-Canel Bermúdez (@DiazCanelB) March 3, 2021
In addition to the three Soberana vaccines, scientists of the Center for Genetic Engineering and Biotechnology (CIGB) are also developing Abdala and Mambisa vaccines.
All five vaccines are protein antigens that do not contain the live virus but contain part of the coronavirus. They work in a similar way and the difference between them is that each one has different formulations. The Soberana vaccines use an antigen obtained from mammalian cells in various formulations, while Mambisa and Abdala use an antigen taken from yeast, also in various formulations.
Just like Soberana 02, Abdala also showed positive results during the first two phases of clinical trials and will advance to the third phase this month.
President Díaz-Canel confirmed the news. In another tweet on March 3, he wrote that “Very soon, phase III clinical trials of Soberana 02 and Abdala will begin in Havana, Santiago de Cuba and Guantánamo, with more than 85,000 volunteers.”
If these vaccines produce fruitful results, Cuba will become the first country in the Latin American and the Caribbean region to develop its own vaccines.
Cuba hopes to produce 100 million doses by the end of this year to vaccinate its entire population of over 11 million people, start exporting to other countries and contribute to the ALBA-TCP’s vaccine bank.