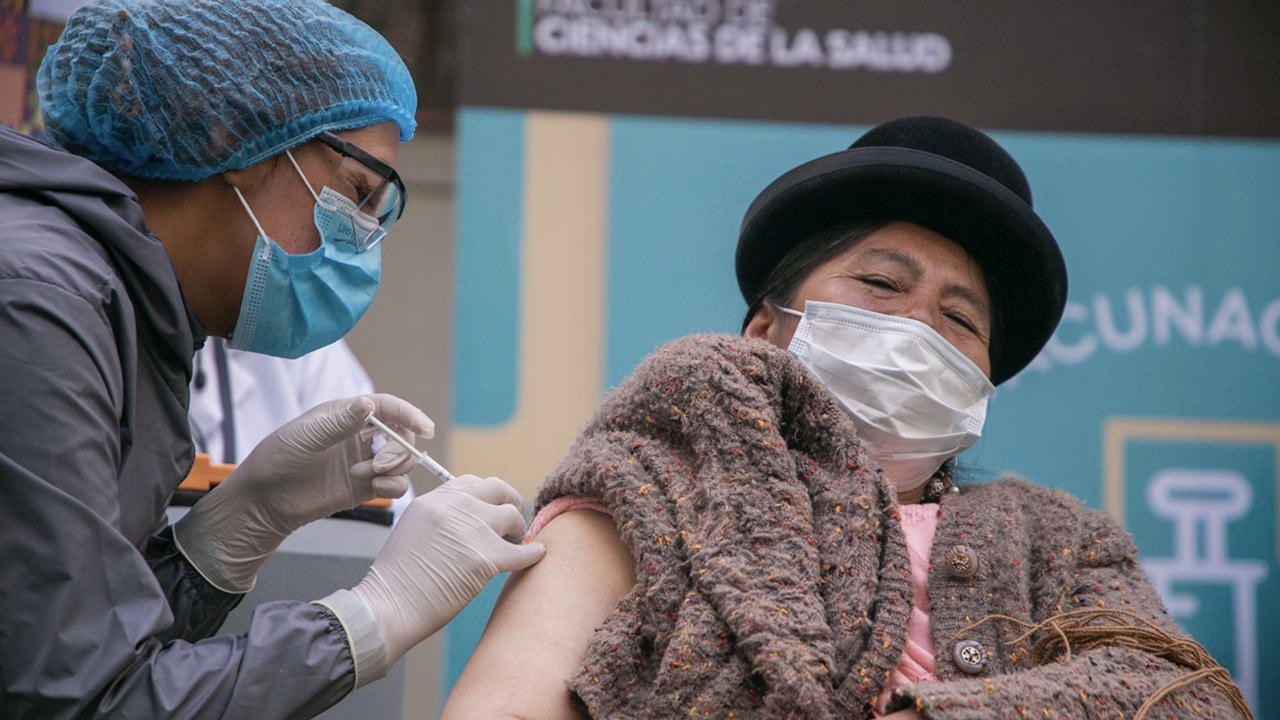
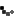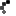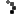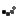The Minister of Foreign Affairs of Bolivia announced on May 11, 2021, that it reached an agreement to import COVID-19 vaccines from the Canadian drug manufacturer Biolyse Pharma, with the potential capacity to produce up to twenty million doses of COVID-19 vaccines per year. But, in order to carry out this measure it is necessary for the Government of Canada to grant a compulsory license, which it has yet to do.
With a population of 11.8 million inhabitants, Bolivia has been able to vaccinate 19.8% of its population with the first dose and fully vaccinate only 6.8% of its population by the end of July 2021. The Bolivian minister stated that “all private or state pharmaceutical companies that have the capacity to produce vaccines should do so, considering that vaccines are ultimately public goods.”
Bolivia notified the World Trade Organization (WTO) of its desire to use the compulsory license mechanism provided for in Article 31 bis of the Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement.
For its part, Biolyse declared its willingness to contribute to vaccination efforts in the world and made public that it had reached an agreement to provide 15 million doses of the Johnson & Johnson single-dose COVID-19 vaccine to Bolivia. The company has the capacity to produce at least 20 million doses per year. However, it cannot move forward until the Canadian Government actually allows it to produce them, or it is granted a voluntary license from the patent holder.
On the other hand, if Canada does not allow Bolivia to import these vaccines under a compulsory license, it would be directly contradicting its own position under the Canadian Access to Medicines Regime (CAMR) and its stand at the current negotiations on a waiver of certain provisions of the TRIPS Agreement, as proposed by South Africa, India and another 63 countries. Canada argues that the existing flexibilities are working “as intended”, citing its own CAMR mechanism as an example.
However, some nations have rejected this argument, stating that a “procedural labyrinth” has been constructed that includes a series of protectionist provisions, preventing the expeditious granting of these compulsory licenses. For example, licenses can only be granted for the products listed in Schedule 1 of the Canadian Patent Law: there are currently no COVID-19 vaccines on that list, and no signs of political will to include them.
Biolyse expresses its agreement to sell vaccines to Bolivia at an estimated manufacturing cost of USD 3.- to USD 4 per dose, more accessible than other vaccines present in the international market on which big pharmaceutical companies hold the patents.
It is not a secret that pharmaceutical companies that developed COVID-19 vaccines received support from public funds. The race to find a cure for COVID-19 saw an estimated expenditure of USD 26 billion in pharmaceutical R&D (Research and Development) in 2020 alone. Just in the US, the Government granted USD 10,000 Billion to the Industry to R&D of the COVID Vaccine.
While they now report extraordinary profits, these companies continue to oppose giving up their patent rights. They argue that low and middle income countries will be slow to establish manufacturing capacity and will end up competing for scarce supplies, which, in their judgment, will hurt production.
The truth is that, as Dr. German Velásquez argued years ago, “we continue in a world where the history of diseases is almost the same, but medicines remain the private property of rich nations and selfish industries” – even in an unprecedented situation such as the COVID-19 pandemic.
Oscar Lanza V. is a member of Peoples Health Movement in Bolivia.